How to use Sogroya® for children
The Sogroya® pen is easy to usea and designed with your lifestyle in mind.

aIn a randomized, crossover device preference and handling study of the Sogroya® pen in 33 adolescents aged 10-17 with growth-related disorders and 37 caregivers aged 18 and older, 99% (69) of participants found the Sogroya® pen easy or very easy to use. After training, users performed simulated injections into a pad or mannequin and then completed the Device Handling and Preference Assessment Questionnaire (DHPAQ).


Important things to know when starting or switching to Sogroya®
Your doctor will specify what dose is right for your child.
If you are switching from a daily GH treatment, make sure at least 8 hours have passed between the last dose of your daily GH treatment and the first dose of Sogroya®.
If you are switching from a weekly GH treatment, you can continue a once-weekly dosing schedule with Sogroya®.
If you are new to GH treatment, start with the Sogroya® dosage strength indicated by your doctor.
Sogroya® is available in 3 different dosing strengths
Your child’s healthcare provider will tell you which Sogroya® pen is right for your child and how to dose it.



Learn to use the pen
New to the Sogroya® pen or looking for a refresher? Our step-by-step tutorial takes you through the process.

Injection instructions
This video, in English with Spanish subtitles, may help you and your child learn more about the injection process.

How to use Sogroya®
The injection process includes 5 steps, from preparing the pen to storing or throwing it away when you’re done with it.b
Please see Instructions for Use for complete instructions.
bDo not share your Sogroya® pen and needles with another person. You may give another person an infection or get an infection from them. Do not use your Sogroya® pen without training from your healthcare provider. Make sure that you are confident in giving an injection with the Sogroya® pen before you start your treatment. If you are blind or have poor eyesight and cannot read the dose counter on the Sogroya® pen, do not use this Sogroya® pen without help. Get help from a person with good eyesight who is trained to use the Sogroya® pen.

Actor portrayal

Pen shown is 10 mg/1.5 mL pen. Please see Instructions for Use for complete instructions for each dosage strength.
1. Prepare your Sogroya® pen
- Wash your hands with soap and water. Then check the name, strength, and colored label on your pen to make sure that it contains Sogroya® in the right strength.
- Pull off the pen cap. Turn the pen upside down 1 or 2 times to check that the Sogroya® in your pen is clear to almost clear and colorless to slightly yellow.
If Sogroya® looks cloudy or you see visible particles, do not use the pen. - Get a new disposable needle and remove the paper tab.c Push the needle straight onto the pen and turn the needle clockwise until it is on tight.
Always use a new needle for each injection. This reduces the risk of contamination, infection, leakage of Sogroya®, and blocked needles leading to incorrect dosing. - Pull off the outer needle cap and throw it away. Pull off the inner needle cap and throw it away too.
cNeedles are sold separately and may require a prescription.

Pen shown is 10 mg/1.5 mL pen. Please see Instructions for Use for complete instructions for each dosage strength.
2. Check the flow with each new pen
- Before using a new pen, check the flow to make sure Sogroya® can flow through the pen and needle.
- Turn the dose selector clockwise one tick mark to select the smallest amount of medicine for the pen.
- Hold the pen with the needle pointing up. Press and hold in the dose button until the dose counter returns to “0.” The “0” must line up with the dose pointer.
- Check that a drop of Sogroya® appears at the needle tip. If no Sogroya® appears, repeat up to 6 times. If you still don’t see a drop of Sogroya®, replace the needle once and try again.
Do not use your pen if a drop of Sogroya® does not appear after changing the needle. Call 1-888-NOVO-444 (1-888-668-6444) for help.
Pen shown is 10 mg/1.5 mL pen. Please see Instructions for Use for complete instructions for each dosage strength.
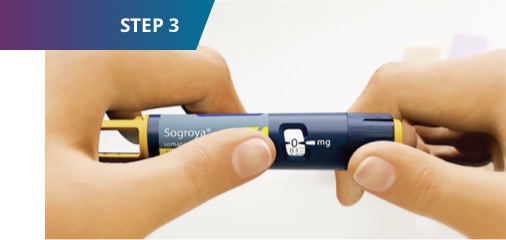
3. Select your dose
- Check that the dose counter is set to “0.”
- Turn the dose selector clockwise to select the dose you need. Always use the dose pointer to select the exact dose. Do not rely on pen clicks.
- If you select the wrong dose, you can turn the dose selector clockwise or counterclockwise to reach the correct dose.
- If there is not enough Sogroya® left in the pen to select your full dose, you can either dispose of it (see step 5) and start over with a new pen or split your dose between your current pen and a new pen. To split your dose, inject the amount left in your current pen. Calculate how much Sogroya® you need to complete your dose, then perform steps 1 through 3 to prepare a new pen and select that amount.
Only split your dose if you have been trained or advised by your healthcare provider on how to do this.
Pen shown is 10 mg/1.5 mL pen. Please see Instructions for Use for complete instructions for each dosage strength.
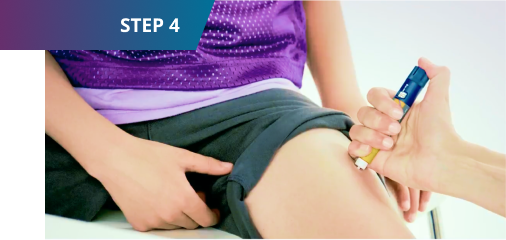
4. Inject your dose
- Sogroya® can be injected under the skin in the upper arms, thigh,
abdomen, or buttocks as instructed by your healthcare provider.
Change the injection site every week. - Wipe the injection site with an alcohol swab and let the area dry.
- Insert the needle into your skin as your healthcare provider has shown you.
Make sure you can see the dose counter. Do not cover it with your fingers, as it could block the injection. - Press and hold down the dose button until the dose counter shows “0.” You may hear or feel a “click.”
- Keep holding down the button with the needle in your skin and slowly count to 6.
- Carefully remove the needle from your skin. You may see a drop of Sogroya® at the needle after injecting. This is normal and does not affect your dose.

Pen shown is 10 mg/1.5 mL pen. Please see Instructions for Use for complete instructions for each dosage strength.
5. After your injection
- Carefully remove the needle from the pen by turning the needle counterclockwise. Immediately place it in an appropriate sharps disposal container.
Always throw away the needle after each injection. - Put the pen cap on your pen after each use to protect Sogroya® from direct light.
- If you won’t reuse the pen, dispose of it in an appropriate sharps disposal container.
Do not throw away loose needles and pens in your household trash.
Frequently asked questions about using Sogroya®
You may have a few questions about using once-weekly Sogroya®. We’ve got the answers you’re looking for.
Yes. You pick the day of the week. The chances of sticking to your once-weekly injection schedule increase by keeping to a consistent routine.
- Set up a consistent routine—routines are an important part of taking Sogroya® as prescribed by your doctor.
- Choose a comfortable spot with the supplies you’ll need nearby.
- Give your child their dose when your child is relaxed and mentally prepared. Singing songs, reading a book, or watching a fun video may help.
Be sure to refer to the instructions that came with your Sogroya® pen.
If within 3 days of missed dose: The dose should be taken as soon as possible, and then resume the usual dosing schedule.
If more than 3 days after missed dose: The dose should be skipped, and the next dose should be taken on the regularly scheduled day.
Store the pen with the cap on, in the carton to protect it from light. Store Sogroya® in a refrigerator between uses. Do not store in the freezer or next to the refrigerator cooling element. Sogroya® may be stored temporarily at room temperature (up to 77 ºF) for up to 72 hours. Return Sogroya® to the refrigerator after room temperature storage.e
eThe pen should be refrigerated (36 °F-46 °F). The pen can be taken in and out of a refrigerator as needed. The pen must be discarded 6 weeks after first use, or if it has been frozen, or in temperatures warmer than 86 °F. Keep Sogroya® away from direct heat and light.
For a complete list of frequently asked questions, please visit our FAQ page.
Get started with Sogroya®
Find out about support programs as you start your GH journey.
Add some creative fun!
Choose from our gallery of free charms and stickers to decorate your Sogroya® pen.

